Fuel Cell Technology
History 1
In the early 1800’s a British scientist discovered the fuel cell effect. He immersed two platinum electrodes in sulfuric acid electrolyte and supplied hydrogen at one electrode and oxygen at the other. The resulting reaction created a current flow between the electrodes. There was no practical application of fuel cells at that time because of high cost and technological problems. In the 1960s, researchers at the University of Vienna demonstrated a fuel cell that was specific for alcohol. This evolved into the present-day cell used in all fuel cell-based breath alcohol measurement instruments.
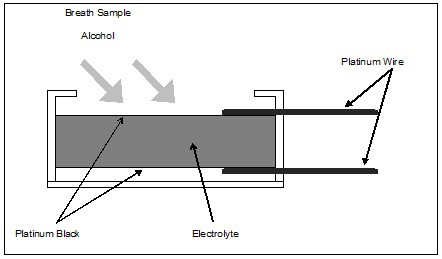
In its simplest form, the alcohol fuel cell consists of a porous, chemically inert layer coated on both sides with finely divided platinum oxide (called platinum black). The manufacturer impregnates the porous layer with an acidic electrolyte solution, and applies platinum wire electrical connections to the platinum black surfaces. The manufacturer mounts the entire assembly in a case, which also includes a gas inlet that allows a breath sample to be introduced. Various manufacturers employ numerous proprietary nuances in their construction. The basic configuration, however, follows that described above and illustrated above.
The chemical reaction that takes place in an alcohol fuel cell converts alcohol to acetic acid. In the process, this conversion produces a fixed number free electrons per molecule of alcohol. This reaction takes place on the upper surface of the fuel cell. H+ ions are freed in the process, and migrate to the lower surface of the cell, where they combine with atmospheric oxygen to form water, consuming one electron per H+ ion in the process. Thus, the upper surface has an excess of electrons, and the lower surface has a corresponding deficiency of electrons. If you connect the two surfaces electrically, a current flows through this external circuit to neutralize the charge. This current is a direct indication of the amount of alcohol consumed by the fuel cell. By measuring the amount of current, you can determine the amount of alcohol in the sample.
The alcohol fuel cells used in Intoximeters, Inc. instruments are highly specific for alcohol on the human breath. The fuel cell produces a linear relationship between current created and alcohol concentration in the breath sample.
Early methods of analyzing the fuel cell output measured the peak rate of reaction and were limited in their ability to deal with the reduced rate of the alcohol oxidation when a series of positive samples were provided in succession.
Current patented methods used for analyzing the output of the cell have solved this problem in that they measure the amount of current generated from the alcohol oxidization reaction as opposed to the rate at which the current was generated. By using this method, the same fuel cell produces results that do not significantly degrade with use and remain within calibration for extended periods of time.
| Strengths of Fuel Cell Based Analyzers | Weaknesses of Fuel Cell Based Analyzers |
| Highly specific for alcohol on the human breath. Does not require multiple sensors and the necessary maintenance to demonstrate that those sensors are performing properly to accurately identify alcohol. |
Analyzes one small fixed volume of breath. It does not analyze changes in the breath sample over the entire submission of the sample.
|
| Linear response to alcohol allows one point calibration of the system. | |
| Low power requirements allows for small packaging of system that produces results as accurate as large desktop instruments. | |
| Battery operated systems available. |
To learn more about the latest technology used for breath alcohol detection click on Fuel Cell White Paper.